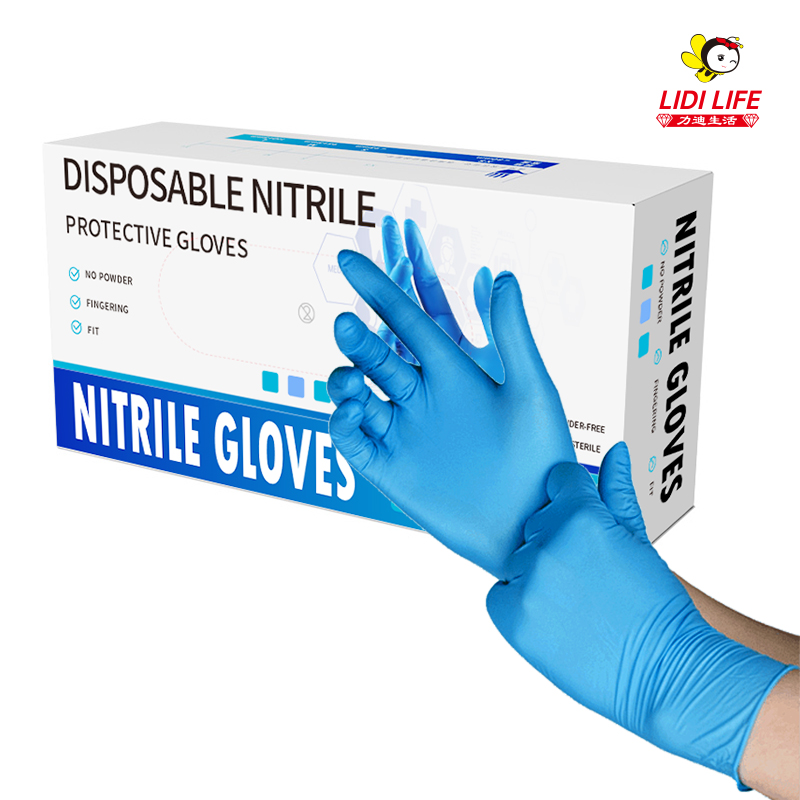
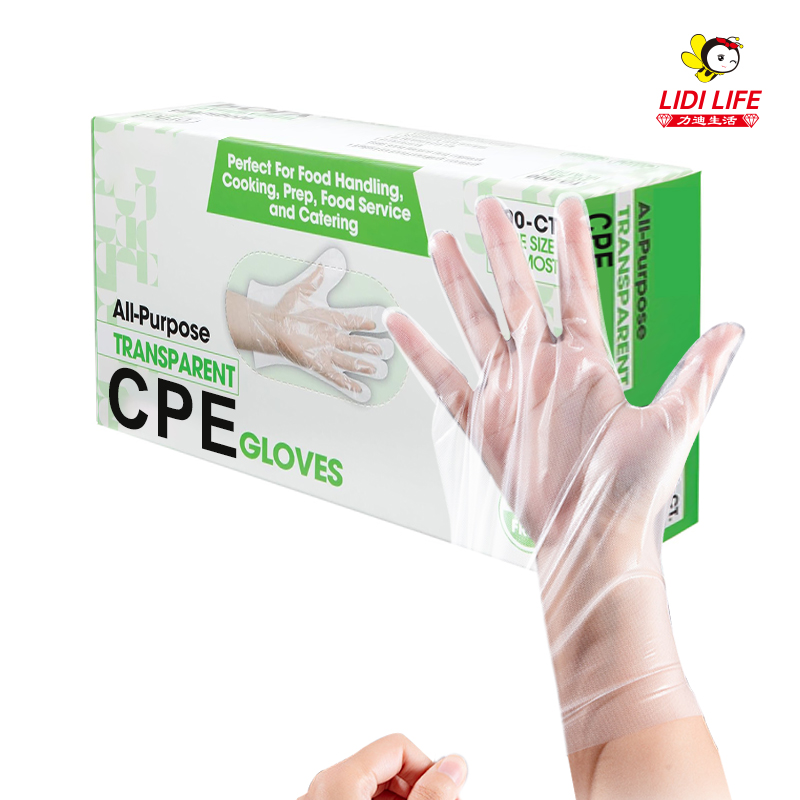
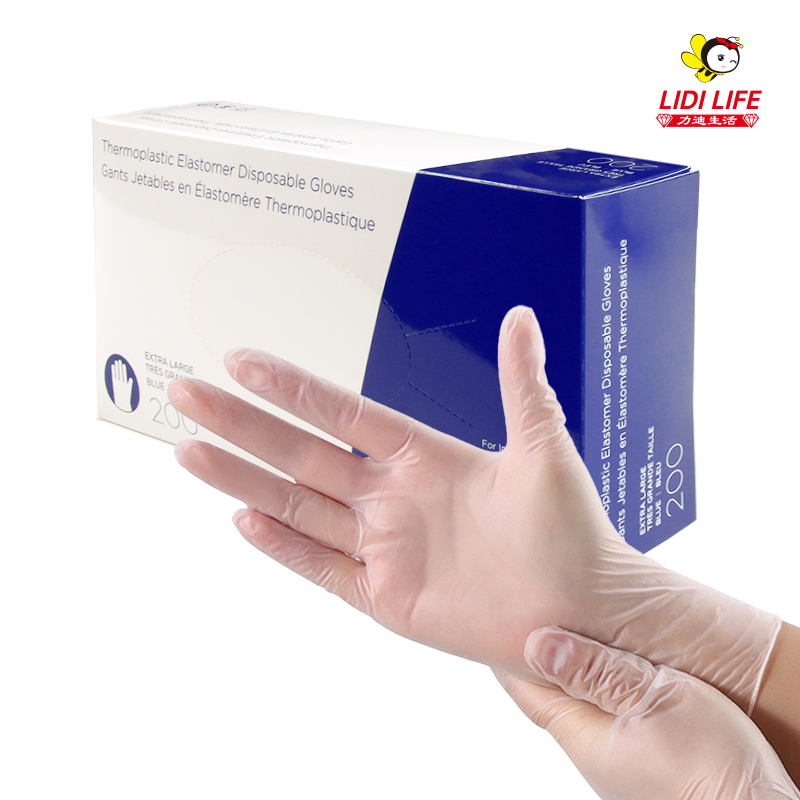
As an enterprise focusing on the production of disposable consumables, Lidi Company has passed a series of qualification certifications such as ISO 9001 quality management system certification, ISO 13485 medical device quality management system certification, CE certification, FDA certification, CFDA certification, EN certification, etc. To ensure the quality of its products and compliance with relevant standards.

Xiamen R&D Center, Fujian, China
Fujian Xiamen R & D Center has the ability and equipment to independently research and develop products, and has a sound R & D system, which can accept your different product orders.

Vietnamese R&D Center
Vietnam R & D Center and Xiamen R & D Center together for customers to develop better products to meet your different needs, we are committed to more professional, more perfect R & D system and goals, to give you the best customer experience.